In recent years, the interest in adipose tissue mesenchymal cell–derived extracellular vesicles (AT-MSC-EVs) has increasingly grown. Numerous articles support the potential of human AT-MSC-EVs as a new therapeutic option for treatment of diverse diseases in the musculoskeletal and cardiovascular systems, kidney, skin, and immune system, among others.
This approach makes use of the molecules transported inside of EVs, which play an important role in cell communication and in transmission of macromolecules. However, to our knowledge, there is no database where essential information about AT-MSC-EVs cargo molecules is gathered for easy reference.
The aim of this paper is to describe the different molecules reported so far in ATMSC- EVs, their main molecular functions, and biological processes in which they are involved. Recently, the presence of 591 proteins and 604 microRNAs (miRNAs) has been described in human AT-MSC-EVs. The main molecular function enabled by both proteins and miRNAs present in human AT-MSC-EVs is the binding function, which supports their role in cell communication.
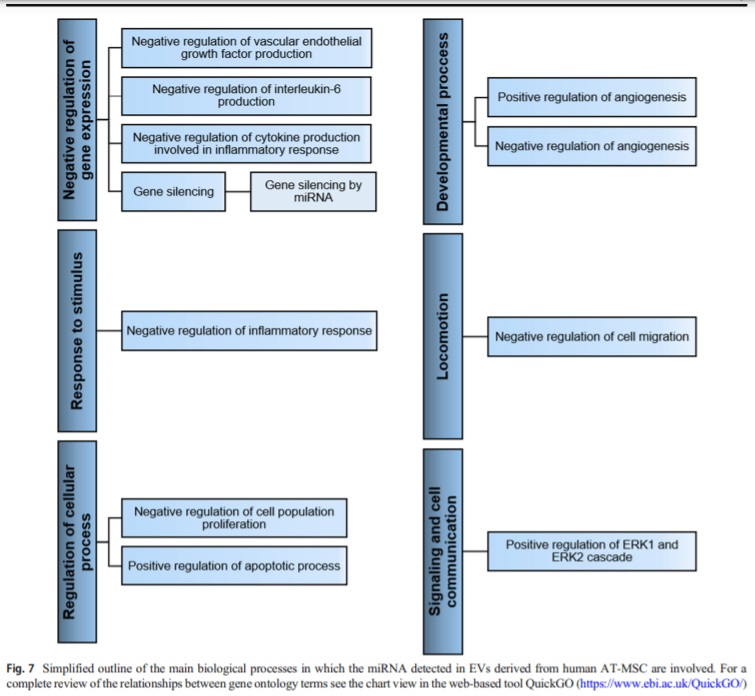
Regarding the biological processes, the proteins detected are mainly involved in signal transduction, while most miRNAs take part in negative regulation of gene expression. The involvement of both molecules in essential biological processes such as inflammation, angiogenesis, cell proliferation, apoptosis and migration, supports the beneficial effects of human ATMSC-EVs observed in both in vitro and in vivo studies, in diseases of the musculoskeletal and cardiovascular systems, kidney, and skin.
Interestingly, the contents of AT-MSC-EVs can be modified by cell stimulation and different cell culture conditions,
such as oxidative stress or hypoxia, to engineer a cargo selection with improved antigenic, anti-inflammatory or immunosuppressive effects. Moreover, it is also possible to enrich specific miRNAs in the cargo via transfection of AT-MSC with lentiviral particles. These modifications have enhanced the positive effects in skin flap survival, immune response, bone regeneration and cancer treatment. This phenomenon opens new avenues to examine the therapeutic potential of AT-MSC-EVs.
In this review are highlighted the therapeutics effects of AT-MSC-EVs related with their participation in relevant biological processes including inflammation, angiogenesis, cell proliferation, apoptosis and migration, among others.

Leave a Reply