
In recent months there have been some major jumps—unprecedented success stories—that indicate our ability to engineer T cells may well have a substantial impact for multiple medical conditions that have not been responsive to conventional therapies or for which there is no available treatment. This can be regarded as the quintessential individualized medicine intervention—specifically modifying the T cells so they can recognize a protein on the surface of a person’s cells— that can markedly dial up or down their immune response. As reviewed in this week’s Nature, there are over 500 clinical trials ongoing that are testing engineered T cells to build upon the first chimeric antigen receptor (CAR-T) cell treatment approved for leukemia 6 years ago. Over 20,000 people have received one of the 6 CAR-T approved therapies for “liquid” blood cancers. Two of the first patients treated with CAR-T 12 years ago for cancer are still in remission. Some of the progress has been highlighted by covers of top tier journals like the one below in Science and Siddhartha Mukherjee’s outstanding new book The Song of the Cell over the past year. It’s pretty clear that in so many ways this field is just getting started.
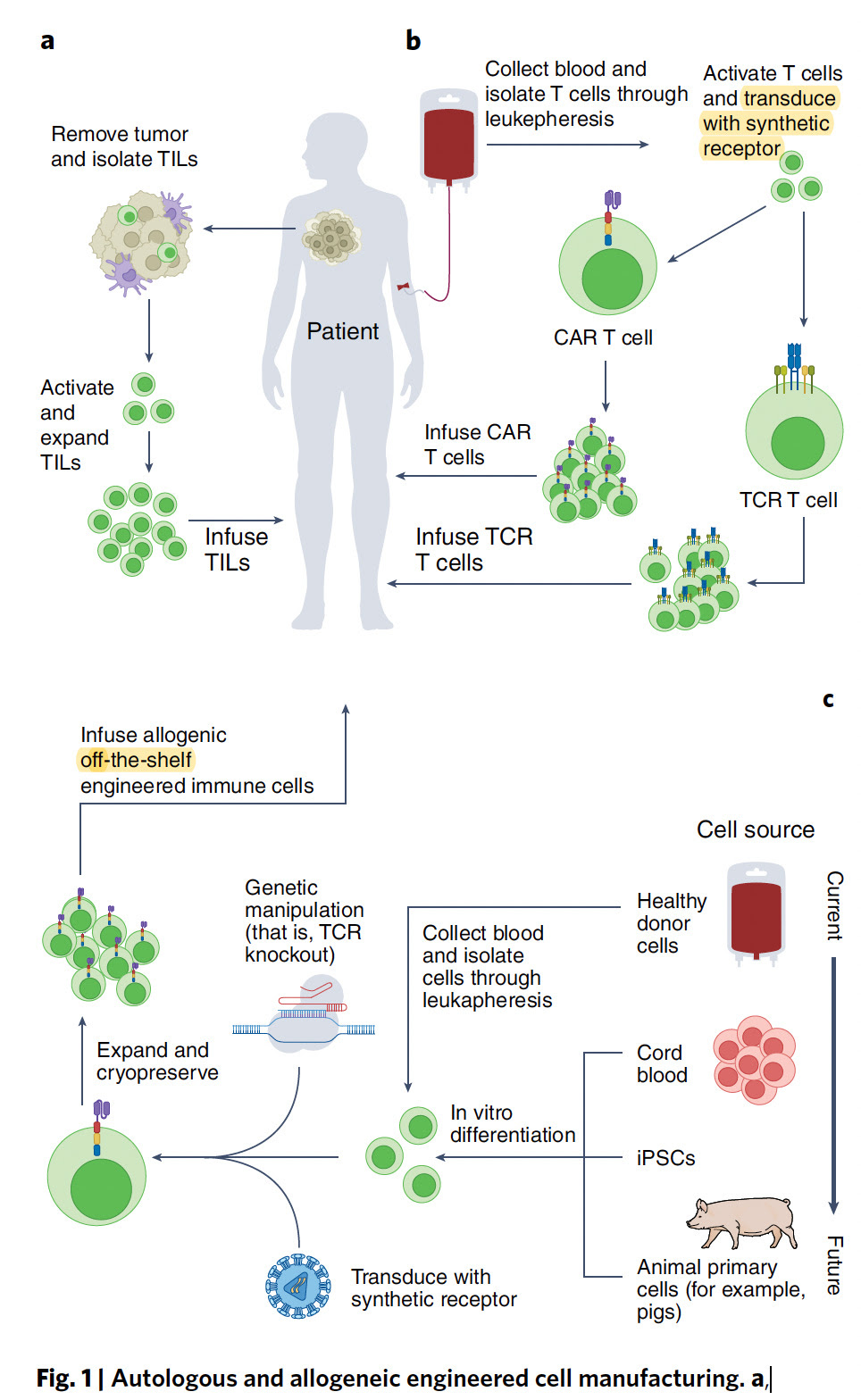
There have been several recent excellent reviews on CAR-T cell engineering for cancer here (with Figure below) and here and here. It is noteworthy to point out there’s not just one type of CAR (it’s never simple!), and I like the summary graphic below that builds on the metaphor with self-driving, armored, and other vehicles. The purpose of this post is not to review the field, but rather to point out the major points of progress that establish new directions and opportunities for this form of treatment.

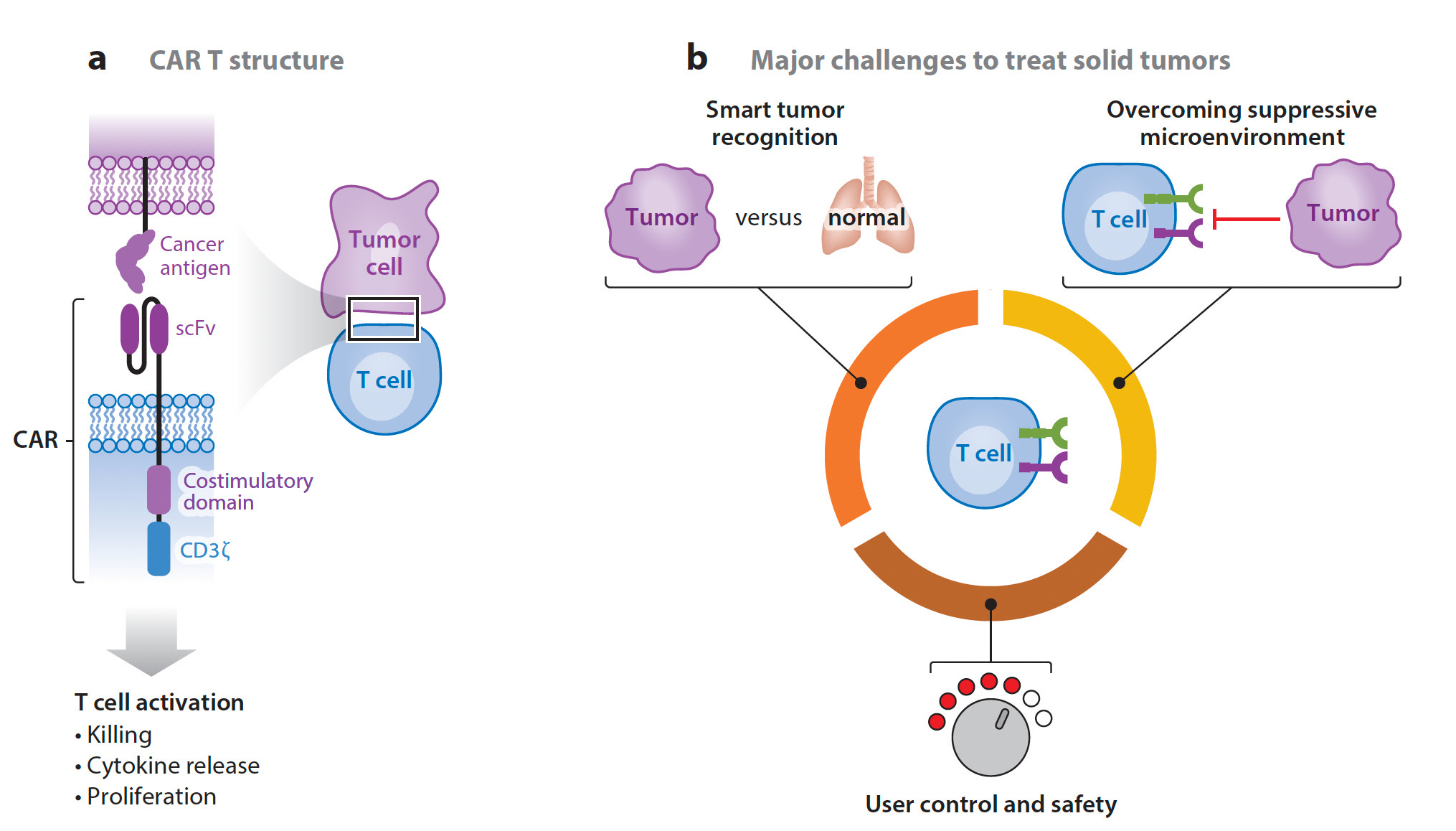
- Solid TumorsAs mentioned, until now the progress for CAR-T in cancer has been for blood malignancies, such as leukemias and multiple myeloma. But we’re now seeing evidence and hope for some of the deadliest types of cancer for which therapy is often ineffective—pancreatic cancer and brain gliomas. As I previously wrote about in a turning point in cancer, a case report of a patient with pancreatic ductal adenocarcinoma. 90% of these cancers have a mutation the KRAS gene, and G12D is the most common one, in about one-third of such cancers. In June 2021, a 71-year-old woman with recurrence of pancreatic cancer that had been extensively treated (including a Whipple surgical procedure, radiation and multiple agent chemotherapy) presented with new lung metastases, received a single infusion of her blood with engineered T cells with 2 genes encoding T cell receptors, that were directed to both the G12D mutation and an HLA allele (HLA-C*08:02) of the immune system. The results of marked regression of her tumor were extraordinary and persistent, although not shared by another patient similarly treated who succumbed from this cancer.In parallel, a highly aggressive form of brain and spinal cord cancer that is universally fatal in children called diffuse intrinsic pontine gliomas (DIPG) and diffuse midline gliomas (DMGs) were recently approached with CAR-T therapy in 4 patients, 3 of whom exhibited marked improvement. This was achieved with both intravenous and directly into the brain, specifically targeting the protein GD2. This work builds on a 2017 paper using CAR-T intravenously for patients with glioblastoma.
It’s still early for CAR-T therapy success for solid cancers as it’s a daunting challenge, as summarized below—differentiating tumor from normal cells and overcoming the cancer’s ability to suppress the heightened immune response, no less simply infiltrating the tumor. There are many new “tricks” being added to the engineering armamentarium as discussed below which may enhance the ultimate chance of prevailing over many solid tumors in the future.
- Autoimmune diseases In contrast to cancer where the goal is to rev up the immune response, here it is just the opposite. The exemplar that this has promise is based on the recent publication of 5 patients with refractory lupus (systemic lupus erythematosus) treated with CAR-T directed against CD-19, documenting success of remission, that was maintained in approximately 1 year follow-up without concomitant drug therapy. Notably this was safe, as the major concern with CAR-T therapy are the cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity sndrome (ICANS), which can be life-threatening. Besides lupus, there are so many autoimmune conditions that can become refractory to medical therapies. Indeed, selective CAR-T intervention has also been recently applied to the experimental model of multiple sclerosis. This paves the way—it can be seen as just the beginning of selectively shutting down the untoward self-directed immune response in the future.
- Fighting fibrosis (scarring) Last year there was a striking experiment in a mouse model of heart failure published in Science whereby the CAR-T cells directed against fibrosis were delivered via an mRNA-nanoparticle package. Remarkably, heart function was restored, as schematically shown below, with the scar-producing cells targeted by fibroblast-activation peptide (FAP) and shown to be cleared by the spleen.Such fibrosis is a root cause of many fatal conditions that can affect most organs including the liver, kidney, lung, bone marrow and heart. The account for more than 40% of deaths in the industrialized world. Again, while this is early work, it lays a foundation for a cell engineering approach to address a major unmet need. On a related front and perhaps more in the over-reaching category is the proposal to use CAR-T to target and reverse senescence as was suggested results from an experimental lung cancer model.
- Marked Enhancement in T cell Engineering The week in Nature Biotechnology a paper was published entitled “Massively parallel knock-in engineering of human T cells” describing a very high efficiency method of genome editing and specific knock-in gene repair. This led to much higher T cell stability and efficacy in multiple cancer models. The use of genome editing to amp up the precision of the T cell receptor attack in cancer, directed against cancer neoantigens, was previously demonstrated in 16 patients with refractory solid tumors .Separately, synthetic biology, via gene switches and circuits, offers another important route for enhanced T cell engineering, as recently reviewed. Just last month two reports, one from Boston University and another from UCSF (graphic below), used this strategy to dual program CAR-T cells to also produce interleukin-2 when they confront a tumor cell, an ancillary path beyond the specific antigen targeted method that can help these cells overcome tumor immune evasion. Methods to override T cell exhaustion and resistance, by disrupting the cell cycle regulators, have also been recently reported. Of course, other cell types can be engineered besides T cells, creating more auxiliary ways to intervene.
- Off the Shelf CAR-TNow for the practical issue that has concerned me for many years, thinking that CAR-T could never become a mainstream therapy. Its cost approximates $500,000 for a single treatment, which doesn’t include the patient’s hospitalization and other required treatments. Perhaps the most important way to dramatically reduce costs is to use off-the shelf (see schematic diagram near top of this post) CAR-T which last week was shown to be feasible and safe in 43 patients with refractory multiple myeloma, with preliminary evidence of efficacy. The off-the shelf path would preempt the very costly, laborious and time consuming work of autologous CAR-T, produced from the patient’s own cells. While it’s still early to determine whether off-the-shelf will be as good as autologous CAR-T, these new findings provide some hope for this major obstacle to be addressed.Frankly I’m struck by the rapid progress that is currently being made with CAR-T, especially in recent weeks as I’ve tired to highlight here in multiple dimensions—the broadening of potential therapeutic indications, the enhancements of the cell engineering, the uniqueness of approaches to refractory conditions for which we have nothing to offer, and the potential for such therapies to be not so impractical and more broadly available. A decade ago I would not have thought this field could take off, and there are still many serious challenges, with limited accessibility and worsening health inequities being high on the list. Nevertheless, I hope you’ll find this summary useful, as a promissory note for a field that looks like it has a chance to get into high gear in the years ahead.
- Source Ground Truths

Leave a Reply